What Happens When Ammonium Chloride Is Heated With Sodium Hydroxide
Ammonium chloride in turn can react with sodium hydroxide to form ammonia gas NH3 water and sodium chloride. NH 4 Cl NaOH NH 3 NaCl H 2 O.
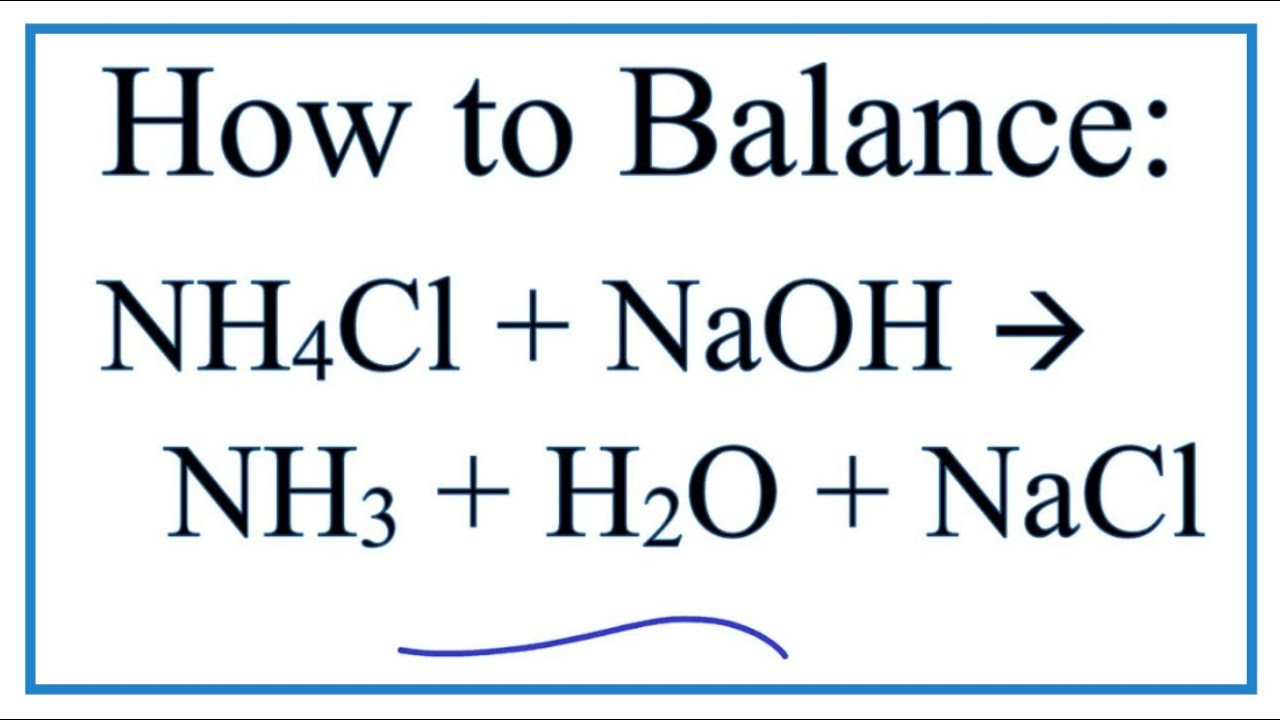
How To Balance Nh4cl Naoh Nh3 H2o Nacl Ammonium Chloride Sodium Hydroxide Youtube
Option D is correct.
. 2 NH 4 Cl Na 2 CO 3 2 NaCl CO 2 H 2 O 2 NH. A 5 by weight solution of ammonium chloride in water has a pH in the range 46 to 60. Practically insoluble in water and ethanol 750 gl TS.
Ammonium chloride and sodium hydroxide. Experiment 1 red litmus paper blue litmus paper aqueous ammonium chloride aqueous sodium hydroxide heat experiment 2 red litmus paper blue litmus paper aqueous ammonium chloride dilute sulphuric acid heat. IronIII nitrate IronIII nitrateIUPAC ID.
The reaction is termed a double displacement reaction. Ammonium chloride reacts with a strong base like sodium hydroxide to release ammonia gas. NH4Cl NaOH NH3 NaCl H2O.
Ammonium chloride appears to sublime upon heating but actually decomposes into ammonia and hydrogen chloride gas. Correct option is D OH. What is the name of Fe no3 2.
Endothermic reactions are chemical reactions wherein the reactants absorb heat from the environment to produce products. Ammonia sodium chloride NaCl and water are given as products when ammonium chloride is heated with aqueous sodium hydroxide NaOH aq. A 3 N H 4 C l N a O H 4 N H 3 N a C l H 2 O.
Ammonium chloride react with sodium hydroxide to produce sodium chloride ammonia and water. As well aqueous NaOH KOH Ba OH 2 are used as alkalis. This is an example of a double displacement reaction.
The reactant ammonium chloride and sodium hydroxide are solid and. In fact this reaction is used to detect the presence of N H 4. Ammonium chloride and sodium hydroxide.
Since sodium is more reactive than iron it displaces the chloride ion from iron to form sodium chloride. The reactant ammonium chloride and sodium hydroxide are solid and. The suppression of the degree of ionisation of a weak electrolyte weak acid or base by an addition of some strong electrolyte having a common ion is called common ion effect.
Kitty Answeregy Expert. Sodium hydroxide NaOH si a strong base that dissociates completely in aqueous solution to form sodium cations which are of no interest here and hydroxide anions. Similarly ammonium chloride also reacts with alkali metal carbonates at elevated temperatures giving ammonia and alkali metal chloride.
When sodium hydroxide and ammonium chloride react ammonia gas is liberated. When ammonium chloride is heated with a solution of sodium hydroxide it leads to the formation of three products namely sodium chloride ammonia and water. Sodium hydroxide is N a O H.
Ammonium chloride reacts with a strong base like sodium hydroxide to release ammonia gas. The gas contains molecules such as ammonia and hydrogen chloride. Some indicators that a chemical reaction has occurred is.
The ammonium reacts with the H O of sodium hydroxide giving ammonia. Ammonium chloride will tend to react with sodium hydroxide to form ammonia water and sodium chloride. What happens when you mix ammonium chloride and sodium hydroxide.
When ammonium chloride is heated with a solution of sodium hydroxide it leads to the formation of three products namely sodium chloride ammonia and water. When Ammonium Chloride is heated with Sodium Hydroxide Ammonia sodium chloride and water is formed. Soluble in hydrochloric acid 70 gl TS and sodium hydroxide 80 gl TS.
Thus concentration ions OH in the solution is considerably reduced and the weak base NH 4. The ammonium chloride undergoes sublimation when it is heated the substance undergoes changes from solid to gas. NH4Cl NaOH NH3 NaCl H2O.
Give the balanced equation for the reaction of ammonium chloride when heated with sodium hydroxide. Aqueous ammonium chloride has weak acidic charactristics. You can test ammonia gas emission from different experiments.
When an ammonium salt is heated with a strong alkali ammonia gas is emitted. When solid ammonium chloride is heated with sodium hydroxide solution a gas ammonia is evolved which is recognised by its strong pungent smell. The reaction is as follows.
The ammonium ion is represented by N H 4. Ammonium chloride will tend to react with sodium hydroxide to form ammonia water and sodium chloride. When sodium hydroxide react with ammonium chloride it liberates.
The products formed are ferric hydroxide and sodium chloride. Ammonium chloride and sodium hydroxide reaction NH 4 Cl NaOH NH 3 NaCl H 2 O. Ammonium Chloride is slightly acidic and Barium Hydroxide very basic so will get an acid-base reaction.
What happens when NaOH is added to nh3. OH becomes a weaker base. When ammonium chloride is heated it undergoes dissociation to form a product such as NH3 and HCl.
The reaction occurs between aqueous solutions of iron II chloride and sodium hydroxide. A white gelatinous precipitate is produced. What happens when Aluminium hydroxide is heated.
A aqueous sodium hydroxide B aqueous sodium sulphate C dilute hydrochloric acid D dilute sulphuric acid 22 The diagrams show two experiments. A solution of a salt or the salt itself is heated in a test-tube with sodium hydroxide solution. -As ammonium chloride is added to the ammonium hydroxide solution the activity of changing or exchanging ions takes place.
Ammonium chloride reacts with a strong base like sodium hydroxide to release ammonia gas. Dissolve 010 g by heating in 5 mL of sodium hydroxide 80 gl TS. To the clear solution add 05 g of ammonium chloride R.
This reaction is used to recover ammonia in the preparation of sodium carbonate from NaCl. As ammonium salt ammonium chloride ammonium nitrate ammonium sulfate and more can be used. What happens when barium hydroxide and ammonium.

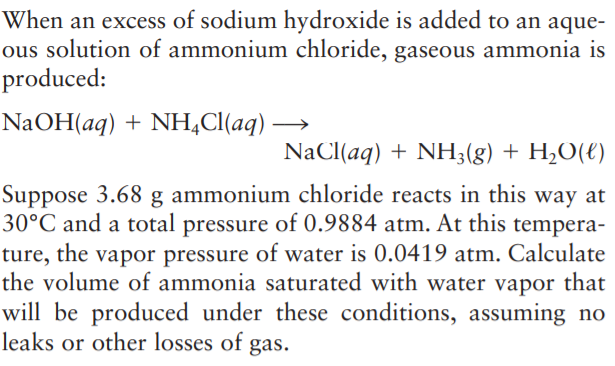
Answered When An Excess Of Sodium Hydroxide Is Bartleby

Double Displacement Reaction Of Ammonium Chloride And Sodium Hydroxide Youtube

Give The Balanced Equation For The Reaction Of Ammonium Chloride When Heated With Sodium Hydroxide
No comments for "What Happens When Ammonium Chloride Is Heated With Sodium Hydroxide"
Post a Comment